Michigan medical device spinoff Tangent Medical Technologies awarded sweeping patent for new IV catheter
The good news keeps coming for University of Michigan medical device spinoff Tangent Medical Technologies, Inc..
After receiving Food and Drug Administration approval for its NovaCath short peripheral intravenous catheter and closing on an $8.6 million round of funding, the company announced Wednesday the issuance of a U.S. patent for the new device that covers a range of incorporated technologies.
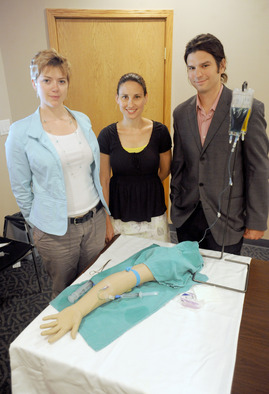
Tangent Medical Technologies co-founders Adrienne Harris, left; Elyse Kemmerer and Steven White stand next to an injectable training arm with an IV system.
Angela J. Cesere | AnnArbor.com
The patent is officially assigned to the University of Michigan, but through a licensing agreement Tangent Medical holds exclusive worldwide rights to the intellectual property.
Tangent vice president for sales and marketing Curtis Bloch said the patent reinforces how impressive the technology is that the company has been developing.
“If you look at how broad the patent is, it speaks to the fact that there are many novel features to this device,” he said.
“… These [IV catheters] first came to market in 1957 and since then no one has thought to turn the fluid path internal to the device. Every big company keeps making these devices with straight fluid paths. Here’s a small innovative company that says ‘why are we doing that?’”
The next step for the company is to commercialize the product and begin to distribute it to hospitals around the country. Bloch said the company is currently scaling up production and will officially commercialize it in the first quarter of 2013.
“We are experiencing strong customer demand for the product right now, and we are engaging hospitals and developing a prioritization list,” he said.
“What I think is that we’re going to be overwhelmed with demand.”

Courtesy Tangent Medical
“There are over 350 million sold in the United States alone,” Bloch said.
“…About 80 percent of people who visit the hospital in an outpatient setting or who are admitted are going to receive a short peripheral IV catheter. These are a high volume disposable item.”
The device has progressed quickly from the University of Michigan Medical Innovation Center to commercialization, a process that took about 40 months. Bloch said the product development life cycle at multi-billion dollar companies could be nearly that long.
“They went through developing the technology, forming the company, designing the product, getting venture backed funding, bringing on the leadership team they have, and getting the product to commercialization in 40 months,” he said. “It’s exceptional.”
Even with that long list, Bloch left off receiving FDA approval for the device and acquiring the patent for the intellectual property.
Two of the major technological breakthroughs that make NovaCath unique and are central to the patent are the internal 180-degree fluid path turn and the stabilization abilities of the device.
“When you show nurses the technology and you show them that the external j-loop is gone, the first thing they all say is, ‘why didn’t I think of that?’” Bloch said.
“It’s great because it saves them and the patient so many hassles.”
The stabilization technology is intended to prevent the needle from moving around inside a patient’s vein once it is inserted. Bloch said stabilization has been a big focus in hospitals for the past five years and that industry trade groups have been emphasizing it as a necessity.
Increased stabilization means decreased patient discomfort.
Bloch said he will be traveling across the country in the coming weeks and months pitching the device to major hospital systems. He said the process for implementation of new IV catheter technologies in hospitals can be four to six months, so he wants to try to start as soon as possible.
Ben Freed covers business for AnnArbor.com. You can sign up here to receive Business Review updates every week. Reach out to Ben at 734-623-2528 or email him at benfreed@annarbor.com. Follow him on twitter @BFreedinA2


Comments
Michigan Man
Fri, Dec 14, 2012 : 12:45 a.m.
Too bad Obamacare will slap a heavy medical device tax on these fine young business and humanitarian types!
trespass
Thu, Dec 13, 2012 : 6 p.m.
What happens when a patient pulls it out themselves? It looks like it would do a lot more harm than a standard IV. You will always have patients who intentionally pull out their IV or get confused and pull it out. Has their been a safety study? When I was a resident at the VA hospital, I saw a trail of blood leading down the hallway, so I followed it. I found a wheelchair bound patient, whose IV line got tangled around the axle of his wheelchair and he was stuck because he couldn't move his wheelchair any further and he was bleeding on the floor. Accidents happen all the time with IV's. Is this one as safe as the standard ones?
Dcam
Fri, Dec 14, 2012 : 4:40 p.m.
I jerked out an IV once, but never again. I never saw a stream of blood squirt so far. The pump pressure was impressive.
Indymama
Thu, Dec 13, 2012 : 5:16 p.m.
Hooray! I hope this will be not only safer,but a more comfortable device!! I hate having IV's...and I've had a few over the years. So I really hope these will be better!!