The Commerce Department is investigating whether imports of personal protective equipment (PPE), medical consumables, and medical devices and equipment have national security implications that may warrant trade action, such as tariffs. This investigation is being conducted under Section 232 of the Trade Expansion Act of 1962 (19 U.S.C. 1862), which is the same investigative mechanism that led to the 2018 tariffs on steel and aluminum. Two trade groups (MDMA and AdvaMed) filed comments cautioning against drastic trade action.
Section 232 Overview
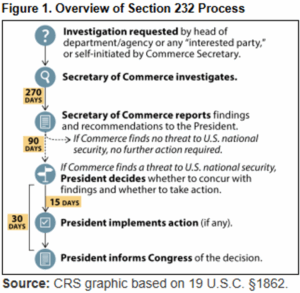
Section 232 allows the President to impose trade restrictions in the name of national security. The statute provides an investigative timeline, outlined in Figure 1 below from the Congressional Research Service (CRS). Once an investigation into imports of identified goods begins, the Commerce Secretary has 270 days to investigate whether such importation may threaten national security and to submit a report to the President. The 270 days often includes a period for public comment. If the report includes findings of a national security threat, the President has 90 days to decide whether to take action, such as imposing tariffs on the identified goods.
Current Section 232 Investigation Into Medical Devices
As published in the Federal Register, “the Secretary of Commerce initiated an investigation to determine the effects on the national security of imports of personal protective equipment (PPE), medical consumables, and medical equipment including devices.” This is the most recent of 12 Section 232 investigations initiated since March 2025, according to a recent report. Other such investigations are directed to copper, timber, semiconductors, trucks, pharmaceuticals, aircraft engines, wind turbines, and more.
This investigation identifies broad categories of goods: “PPE” includes surgical masks, gloves, and gowns; “medical consumables” include “single-use or short-term-use items used for patient diagnosis, treatment, and prevention of conditions”; and “medical devices” include “any instrument, apparatus, or machine used in the diagnosis, monitoring, or treatment of medical conditions.”
Comments From Trade Groups
At least two trade groups, MDMA and AdvaMed, have submitted comments on the investigation.
The Medical Device Manufacturers Association (MDMA) submitted comments advising against imposing Section 232 tariffs. MDMA warned that “extending Section 232 national security tariffs to medical consumables and medical devices/equipment would needlessly disrupt a globally integrated industry that already meets domestic demand, complies with rigorous regulatory standards, and supports American innovation and jobs.”
The Advanced Medical Technology Association (AdvaMed) also submitted comments advising against blanket Section 232 tariffs. AdvaMed included 11 policy recommendations, including requests for low- or no-tariff arrangements with Europe, Japan, the UK, Mexico, Canada, Costa Rica, and the Dominican Republic.
Current Status
The period for public comments is now closed. The Commerce Department has until the end of May 2026 to provide a report to the President with findings and possible recommendations. If the report includes findings of a threat to national security, the President will have up to 90 days to decide whether to take action.
Tags
commerce, import, imports, med device, medical device, national security, section 232, tariff
